Abstract
Background: Eculizumab, an anti-C5 antibody, was approved for the treatment of patients (pts) with symptomatic paroxysmal nocturnal hemoglobinuria (PNH) in 2007 and has been the standard of care for over a decade. However, published data on real-world outcomes of eculizumab-treated pts with PNH are limited. The aim of this study was to describe the clinical profile of pts with PNH treated with eculizumab by characterizing their short- and long-term laboratory and clinical outcomes.
Methods: This retrospective study (Versmold et al, Blood 2020) used preexisting medical records of eculizumab-treated pts with PNH (treatment duration ≥24 weeks [wks]) treated at the University Hospital Essen, Germany prior to April 2018. Anonymized data were collected via electronic case report forms. Laboratory data were extracted from the hospital computer system. Lactate dehydrogenase (LDH), hemoglobin, absolute reticulocyte count (ARC), and bilirubin profiles were assessed at baseline (12 months before treatment) and during the treatment phase (up to 13.2 years [yrs] follow-up). Breakthrough hemolysis (BTH) was defined as ≥1 new symptom or sign of intravascular hemolysis (including fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia [hemoglobin <10 g/dL], major adverse vascular event [including thrombosis], dysphagia, or erectile dysfunction in the presence of elevated LDH [≥2 × the upper limit of normal (ULN)] after reduction of LDH to ≤1.5 × ULN). Extravascular hemolysis was defined as persistence of reticulocytes >100 × 10 9/L with bilirubin >1 × ULN and positive direct Coombs test or reticulocytes >100 × 10 9/L with bilirubin >1 × ULN and ≥1 positive C3c or C3d test. Complete hematologic response was zero blood transfusions with hemoglobin ≥12 g/dL and LDH ≤1.5 × ULN and major hematologic response was zero blood transfusions with hemoglobin ≥12 g/dL and LDH >1.5 × ULN within any 24-wk window (Risitano et al, Front Immunol 2019). Transfusion-dependence was ≥2 blood transfusions within any 24-wk period. Pts transferred from other centers or within 24 wks of treatment were excluded due to missing baseline data.
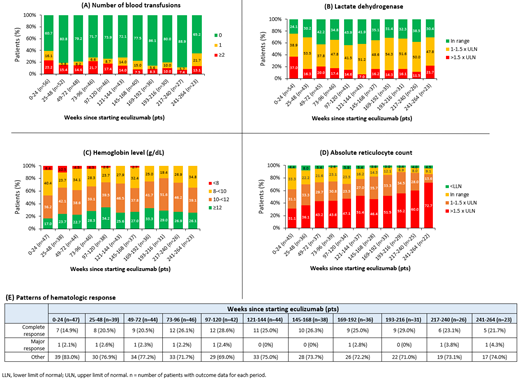
Results: The study included 56 pts with PNH (mean age: 42.9 yrs [± 17.6]; 46.4% female) treated with eculizumab for ≥24 wks (mean follow-up: 5.24 yrs [± 3.25]) during the study period. The median duration from diagnosis to starting eculizumab was 1.57 yrs. Overall, 18 pts (32.1%) had aplastic anemia at diagnosis, 10 (17.9%) had symptoms of high disease activity, and 34 (60.7%) had a blood transfusion in the prior 12 months. The most reported disease-related symptoms at baseline were anemia (28.6%), fatigue (26.8%), thrombosis (21.4%), dyspnea (17.9%), dysphagia (10.7%), erectile dysfunction (10.0%), kidney complications (8.9%), abdominal pain (8.9%), and hemoglobinuria (7.1%). Mean hemoglobin (n=44) was 9.67 g/dL [± 2.06] and LDH in the past 12 months (n=47) was 1480 U/L [± 1010]. During the first 24-wk treatment phase, 37% (20/54) of pts had LDH >1.5 × ULN, 31% (14/45) had ARC >1.5 × ULN, and 17% (8/47) had hemoglobin ≥12 g/dL (Figure). Among pts with response data, 15% (7/47) had complete hematologic response and 2% (1/47) had major hematologic response within 24 wks. Documented BTH with symptoms occurred in 11% (6/56). Moreover, 23% (13/56) of pts were transfusion-dependent, increasing to 39% (22/56) when including pts who had ≥1 transfusion during the first 24 wks of treatment. Six pts (11%) received a higher-than-labeled dose (600 mg intravenous [IV] weekly for 4 wks, 900 mg IV 1 wk later, then 900 mg IV every 2 wks thereafter) of eculizumab.
Over the long term (ie, between 25 and 246 wks), 11.1-34.7% of pts received blood transfusions and 7.0-21.7% had LDH >1.5 × ULN in any 24-wk window; whereas 36.1-72.7% had ARC >1.5 × ULN (Figure). Moreover, 65.8-77.3% of pts had hemoglobin <12 g/dL within any 24-wk period and 69.0-77.2% did not meet the criteria for major or complete hematologic response during any 24-wk period from wks 25 to 246. During the treatment phase, no meningococcal infections were reported.
Conclusions: In this long-term real-world study, a considerable proportion of pts with PNH treated with eculizumab did not achieve optimal clinical outcomes with an ongoing burden of disease (ie, low hemoglobin level with high reticulocyte count due to extravascular hemolysis, BTH, etc.). Future exploration of other therapies that improve pt outcomes could help to address remaining unmet medical needs.
Alashkar: Alexion: Honoraria; Novartis: Honoraria; BMS/Celgene: Honoraria; Bluebird Bio: Honoraria. Ofori-Asenso: F. Hoffmann-La Roche Ltd: Current Employment. Xu: F. Hoffmann-La Roche AG: Current Employment. Liu: Genesis Research: Current Employment. Katz: F. Hoffman-La Roche Ltd: Current Employment. Shang: F. Hoffman-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Roeth: Apellis Pharmaceuticals: Consultancy, Honoraria; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Bioverativ, a Sanofi company: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Kira: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal